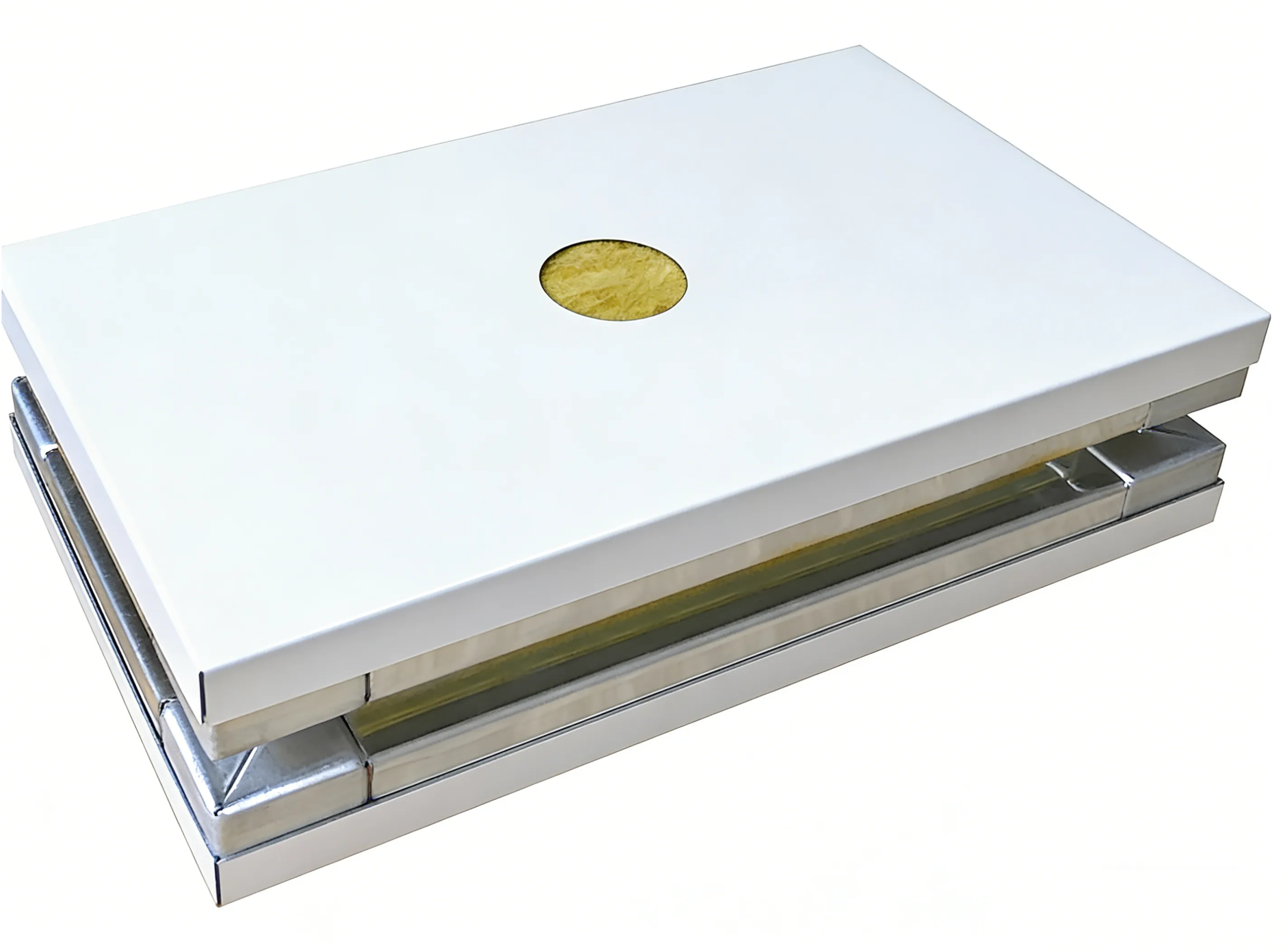
 Fire Safety Priority
Fire Safety Priority
Fireproof Rockwool Cleanroom Panel
- A1 non-combustible fireproof
- H₂O₂ resistant
- High strength & stable structure
Solves: Fire code overrides all other considerations
 High Cleanliness & Fire Rated
High Cleanliness & Fire Rated
Magnesium Cleanroom Panel
- No fiber shedding
- B-s1,d0 fire performance
- High rigidity
Solves: Non-combustible fire safety, cleanliness and solid structural strength are all required
 Thermal Insulation
Thermal Insulation
PU/EPS/PIR Cleanroom Panel
- Superior Thermal Insulation
- Lightweight & easy installation
- Energy-efficiency
Solves: Economic advantages than rockwool and magnesium systems
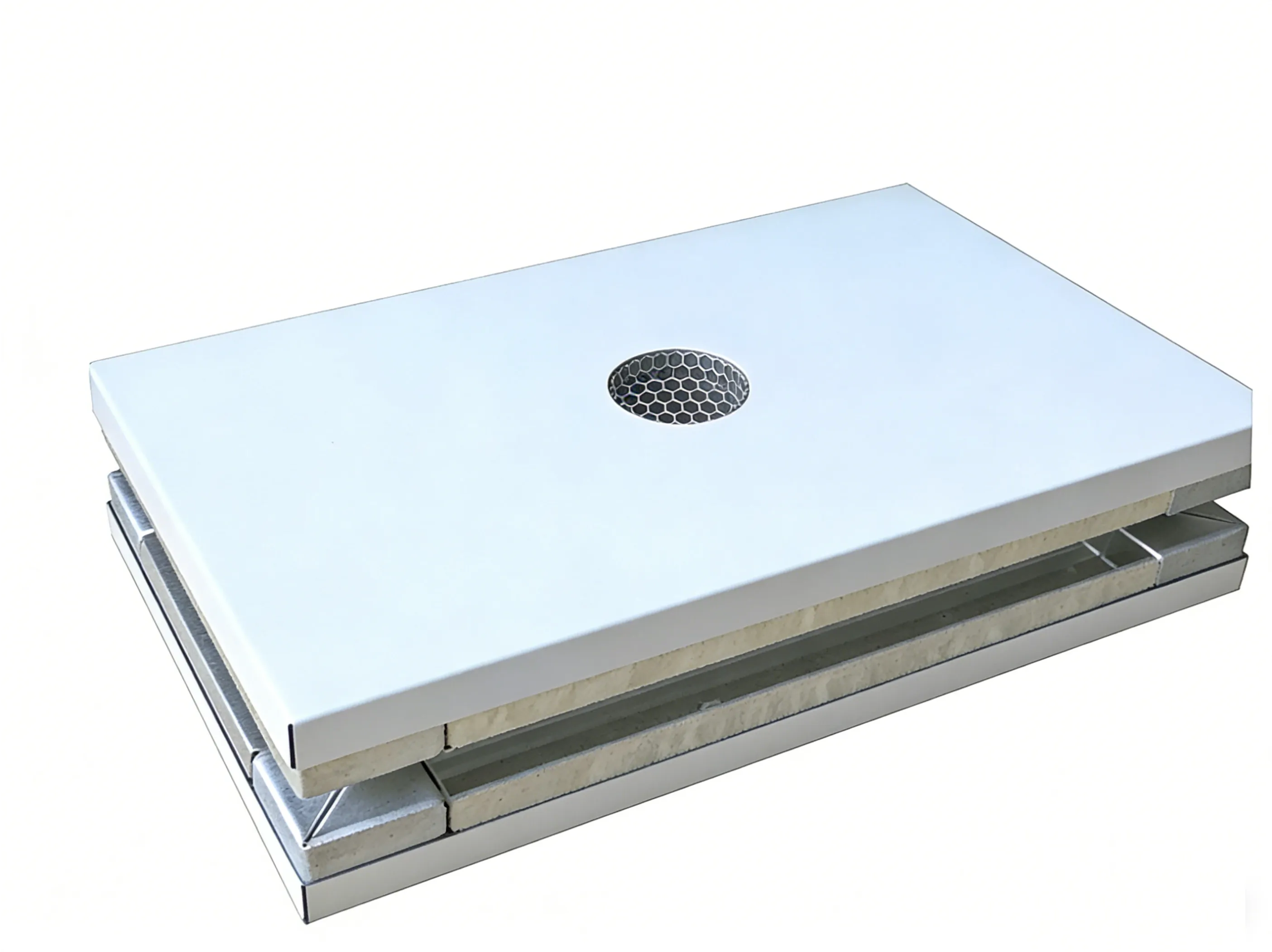 Lightweight Rigidity
Lightweight Rigidity
Aluminum/Paper Honeycomb Panel
- Lightweight core & Modularity
- Smooth surface
- Low particle accumulation
Solves: Structural load limits and fast installation
 Hygiene & Durability
Hygiene & Durability
HPL/Stainless-steel Panel
- VHP Chemical resistance
- Certified Anti-scratch
- Long service life
Solves: Maintenance concerns and surface-lifecycle decision
 Precision Manufacturing
Precision Manufacturing
Machine-made Sandwich Panel
- ±0.1mm accuracy
- Continuous production
- Uniform density
Solves: Inconsistent quality
 High Load & Airtight
High Load & Airtight
Ceiling Panels
- High load stability
- Airtight joint design
- Rapid reconfiguration
Solves: Structural sagging in large cleanrooms & High cost of future renovations
Products
faq
-
What types of cleanroom wall panels are available, and how do they differ in performance and application?
-
How do I choose between fire-rated panels and insulation-focused panels?
-
Are all panels suitable for different cleanroom iso classes?
-
Are hollow or honeycomb panels suitable for concealing utilities?
-
What is the difference between handmade and machine-made partition panels?









